
-
 Lens cruise into French Cup quarters, Endrick sends Lyon through
Lens cruise into French Cup quarters, Endrick sends Lyon through
-
No.1 Scheffler excited for Koepka return from LIV Golf

-
 Curling quietly kicks off sports programme at 2026 Winter Olympics
Curling quietly kicks off sports programme at 2026 Winter Olympics
-
Undav pokes Stuttgart past Kiel into German Cup semis

-
 Germany goalkeeper Ter Stegen to undergo surgery
Germany goalkeeper Ter Stegen to undergo surgery
-
Bezos-led Washington Post announces 'painful' job cuts

-
 Iran says US talks are on, as Trump warns supreme leader
Iran says US talks are on, as Trump warns supreme leader
-
Gaza health officials say strikes kill 24 after Israel says officer wounded

-
 Empress's crown dropped in Louvre heist to be fully restored: museum
Empress's crown dropped in Louvre heist to be fully restored: museum
-
UK PM says Mandelson 'lied' about Epstein relations

-
 Shai to miss NBA All-Star Game with abdominal strain
Shai to miss NBA All-Star Game with abdominal strain
-
Trump suggests 'softer touch' needed on immigration

-
 From 'flop' to Super Bowl favorite: Sam Darnold's second act
From 'flop' to Super Bowl favorite: Sam Darnold's second act
-
Man sentenced to life in prison for plotting to kill Trump in 2024

-
 Native Americans on high alert over Minneapolis crackdown
Native Americans on high alert over Minneapolis crackdown
-
Dallas deals Davis to Wizards in blockbuster NBA deal: report

-
 Russia 'no longer bound' by nuclear arms limits as treaty with US ends
Russia 'no longer bound' by nuclear arms limits as treaty with US ends
-
Panama hits back after China warns of 'heavy price' in ports row

-
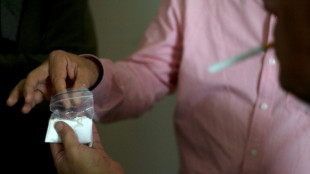 Strike kills guerrillas as US, Colombia agree to target narco bosses
Strike kills guerrillas as US, Colombia agree to target narco bosses
-
Wildfire smoke kills more than 24,000 Americans a year: study

-
 Telegram founder slams Spain PM over under-16s social media ban
Telegram founder slams Spain PM over under-16s social media ban
-
Curling kicks off sports programme at 2026 Winter Olympics

-
 Preventative cholera vaccination resumes as global supply swells: WHO
Preventative cholera vaccination resumes as global supply swells: WHO
-
Wales' Macleod ready for 'physical battle' against England in Six Nations

-
 Xi calls for 'mutual respect' with Trump, hails ties with Putin
Xi calls for 'mutual respect' with Trump, hails ties with Putin
-
'All-time great': Maye's ambitions go beyond record Super Bowl bid

-
 Shadow over Vonn as Shiffrin, Odermatt headline Olympic skiing
Shadow over Vonn as Shiffrin, Odermatt headline Olympic skiing
-
US seeks minerals trade zone in rare Trump move with allies

-
 Ukraine says Abu Dhabi talks with Russia 'substantive and productive'
Ukraine says Abu Dhabi talks with Russia 'substantive and productive'
-
Brazil mine disaster victims in London to 'demand what is owed'

-
 AI-fuelled tech stock selloff rolls on
AI-fuelled tech stock selloff rolls on
-
Russia vows to act 'responsibly' as nuclear pact ends with US

-
 White says time at Toulon has made him a better Scotland player
White says time at Toulon has made him a better Scotland player
-
Washington Post announces 'painful' job cuts

-
 All lights are go for Jalibert, says France's Dupont
All lights are go for Jalibert, says France's Dupont
-
Artist rubs out Meloni church fresco after controversy

-
 Palestinians in Egypt torn on return to a Gaza with 'no future'
Palestinians in Egypt torn on return to a Gaza with 'no future'
-
US removing 700 immigration officers from Minnesota

-
 Who is behind the killing of late ruler Gaddafi's son, and why now?
Who is behind the killing of late ruler Gaddafi's son, and why now?
-
Coach Thioune tasked with saving battling Bremen

-
 Russia vows to act 'responsibly' once nuclear pact with US ends
Russia vows to act 'responsibly' once nuclear pact with US ends
-
Son of Norway's crown princess admits excesses but denies rape

-
 US calls for minerals trade zone in rare move with allies
US calls for minerals trade zone in rare move with allies
-
Vowles dismisses Williams 2026 title hopes as 'not realistic'

-
 'Dinosaur' Glenn chasing skating gold in first Olympics
'Dinosaur' Glenn chasing skating gold in first Olympics
-
Gaza health officials say strikes kill 23 after Israel says shots wounded officer

-
 Italy foils Russian cyberattacks targeting Olympics
Italy foils Russian cyberattacks targeting Olympics
-
Stocks stabilise after Wall St AI-fuelled sell-off

-
 Figure skating favourite Malinin feeling 'the pressure' in Milan
Figure skating favourite Malinin feeling 'the pressure' in Milan
-
Netflix film probes conviction of UK baby killer nurse


BioNxt Launches 15-day Sublingual Cladribine Dosing Optimization Study in Preparation for Human Bioequivalence Study
VANCOUVER, BC / ACCESS Newswire / October 21, 2025 / BioNxt Solutions Inc. ("BioNxt" or the "Company") (CSE:BNXT)(OTCQB:BNXTF)(FSE:BXT), a bioscience innovator specializing in next-generation drug delivery technologies, is pleased to announce the launch of an important large-mass animal bioequivalence study for its lead product, BNT23001, a proprietary sublingual Cladribine formulation for the treatment of multiple sclerosis ("MS"). This is the final animal study to generate dosing parameters for the Company's human comparative bioequivalence study planned for early 2026.
"The Company has already confirmed dosage bioequivalence in small-mass (40kgs) animal study will provide valuable insight into the appropriate sublingual drug load for humans," Hugh Rogers, CEO of BioNxt. "This large-mass animal study is expected to increase the formula precision in our human study and is designed to generate comparative drug absorption results between the Company's sublingual formulation versus the name brand tablet formulation. Optimization of drug load per dose and potential super bioavailability are key pieces of information that will guide the final clinical planning for our upcoming comparative human bioequivalence study."
The large-mass animal crossover bioequivalence study will commence in the next two to three weeks and will be carried out over the following 15 days. The study is expected to be completed in November with results available in December.
BNT23001 is an orally dissolvable thin-film formulation of cladribine, a well-established immunomodulatory compound used for the treatment of MS. Delivered sublingually, the formulation is designed for improved bioavailability, faster onset of action, and enhanced patient compliance, particularly in populations affected by dysphagia or seeking non-invasive alternatives to traditional tablets or injections. Preclinical studies, as previously reported, have demonstrated high absorption rates and bioequivalence to existing oral therapies as well as zero indications of toxicity.
The patent nationalization process is underway in key global markets, including the European Union, Canada, Australia, Eurasia, New Zealand, and Japan, as well a Track One priority filing in the United States. Both the European Patent Office and the Eurasian Patent Office have issued favorable communications, including notice of intentions to grant. Novelty, inventive step, and industrial applicability were fully accepted.
About BioNxt Solutions Inc.
BioNxt Solutions Inc. is a bioscience innovator focused on next-generation drug delivery platforms, diagnostic screening systems, and active pharmaceutical ingredient development. Its proprietary platforms include sublingual thin films, transdermal patches, oral tablets, and a new targeted chemotherapy platform designed to deliver cancer drugs directly to tumors while reducing side effects.
With research and development operations in North America and Europe, BioNxt is advancing regulatory approvals and commercialization efforts, primarily focused on European markets. BioNxt is committed to improving healthcare by delivering precise, patient-centric solutions that enhance treatment outcomes worldwide.
BioNxt is listed on the Canadian Securities Exchange: BNXT, OTC Markets: BNXTF and trades in Germany under WKN: A3D1K3. To learn more about BioNxt, please visit www.bionxt.com.
Investor Relations & Media Contact
Hugh Rogers, Co-Founder, CEO and Director
Email: [email protected]
Phone: +1 604.250.6162
Web: www.bionxt.com
LinkedIn: https://www.linkedin.com/company/bionxt-solutions
Instagram: https://www.instagram.com/bionxt
Cautionary Statement Regarding "Forward-Looking" Information
This press release contains "forward-looking information" and "forward-looking statements" within the meaning of applicable Canadian securities laws (collectively, "forward-looking information"). Such information may include, but is not limited to, statements regarding: the anticipated grant, scope, and timing of European, Eurasian, and other international patent rights; the Company's plans for additional national filings; the development, clinical evaluation, regulatory approval, and commercialization of the Company's Cladribine sublingual thin-film (BNT23001) for multiple sclerosis; the strategic importance of intellectual property protection; the timing, cost, and outcome of preclinical and clinical studies; and the potential application of BioNxt's sublingual thin-film drug delivery platform across additional therapeutic areas.
Forward-looking information is based on management's current expectations, assumptions, estimates, and projections as of the date of this press release. Such statements are subject to inherent risks and uncertainties, many of which are beyond the Company's control, that could cause actual results, performance, or achievements to differ materially from those expressed or implied. These risks and uncertainties include, but are not limited to: outcomes of patent examination and prosecution processes; changes in regulatory requirements or legal frameworks; the results, timing, and costs of preclinical and clinical studies; scalability and reproducibility of manufacturing processes; the availability of strategic partnerships and funding; and broader economic, financial, or geopolitical factors.
Readers are cautioned not to place undue reliance on forward-looking information. Although the Company believes the expectations and assumptions underlying such information are reasonable, there can be no assurance that they will prove to be correct. Except as required under applicable securities laws, BioNxt undertakes no obligation to update or revise any forward-looking information, whether as a result of new information, future events, or otherwise.
SOURCE: BioNxt Solutions Inc.
View the original press release on ACCESS Newswire
B.Shevchenko--BTB


