
-
 Lens cruise into French Cup quarters, Endrick sends Lyon through
Lens cruise into French Cup quarters, Endrick sends Lyon through
-
No.1 Scheffler excited for Koepka return from LIV Golf

-
 Curling quietly kicks off sports programme at 2026 Winter Olympics
Curling quietly kicks off sports programme at 2026 Winter Olympics
-
Undav pokes Stuttgart past Kiel into German Cup semis

-
 Germany goalkeeper Ter Stegen to undergo surgery
Germany goalkeeper Ter Stegen to undergo surgery
-
Bezos-led Washington Post announces 'painful' job cuts

-
 Iran says US talks are on, as Trump warns supreme leader
Iran says US talks are on, as Trump warns supreme leader
-
Gaza health officials say strikes kill 24 after Israel says officer wounded

-
 Empress's crown dropped in Louvre heist to be fully restored: museum
Empress's crown dropped in Louvre heist to be fully restored: museum
-
UK PM says Mandelson 'lied' about Epstein relations

-
 Shai to miss NBA All-Star Game with abdominal strain
Shai to miss NBA All-Star Game with abdominal strain
-
Trump suggests 'softer touch' needed on immigration

-
 From 'flop' to Super Bowl favorite: Sam Darnold's second act
From 'flop' to Super Bowl favorite: Sam Darnold's second act
-
Man sentenced to life in prison for plotting to kill Trump in 2024

-
 Native Americans on high alert over Minneapolis crackdown
Native Americans on high alert over Minneapolis crackdown
-
Dallas deals Davis to Wizards in blockbuster NBA deal: report

-
 Russia 'no longer bound' by nuclear arms limits as treaty with US ends
Russia 'no longer bound' by nuclear arms limits as treaty with US ends
-
Panama hits back after China warns of 'heavy price' in ports row

-
 Strike kills guerrillas as US, Colombia agree to target narco bosses
Strike kills guerrillas as US, Colombia agree to target narco bosses
-
Wildfire smoke kills more than 24,000 Americans a year: study

-
 Telegram founder slams Spain PM over under-16s social media ban
Telegram founder slams Spain PM over under-16s social media ban
-
Curling kicks off sports programme at 2026 Winter Olympics

-
 Preventative cholera vaccination resumes as global supply swells: WHO
Preventative cholera vaccination resumes as global supply swells: WHO
-
Wales' Macleod ready for 'physical battle' against England in Six Nations

-
 Xi calls for 'mutual respect' with Trump, hails ties with Putin
Xi calls for 'mutual respect' with Trump, hails ties with Putin
-
'All-time great': Maye's ambitions go beyond record Super Bowl bid

-
 Shadow over Vonn as Shiffrin, Odermatt headline Olympic skiing
Shadow over Vonn as Shiffrin, Odermatt headline Olympic skiing
-
US seeks minerals trade zone in rare Trump move with allies

-
 Ukraine says Abu Dhabi talks with Russia 'substantive and productive'
Ukraine says Abu Dhabi talks with Russia 'substantive and productive'
-
Brazil mine disaster victims in London to 'demand what is owed'

-
 AI-fuelled tech stock selloff rolls on
AI-fuelled tech stock selloff rolls on
-
Russia vows to act 'responsibly' as nuclear pact ends with US

-
 White says time at Toulon has made him a better Scotland player
White says time at Toulon has made him a better Scotland player
-
Washington Post announces 'painful' job cuts

-
 All lights are go for Jalibert, says France's Dupont
All lights are go for Jalibert, says France's Dupont
-
Artist rubs out Meloni church fresco after controversy

-
 Palestinians in Egypt torn on return to a Gaza with 'no future'
Palestinians in Egypt torn on return to a Gaza with 'no future'
-
US removing 700 immigration officers from Minnesota

-
 Who is behind the killing of late ruler Gaddafi's son, and why now?
Who is behind the killing of late ruler Gaddafi's son, and why now?
-
Coach Thioune tasked with saving battling Bremen

-
 Russia vows to act 'responsibly' once nuclear pact with US ends
Russia vows to act 'responsibly' once nuclear pact with US ends
-
Son of Norway's crown princess admits excesses but denies rape

-
 US calls for minerals trade zone in rare move with allies
US calls for minerals trade zone in rare move with allies
-
Vowles dismisses Williams 2026 title hopes as 'not realistic'

-
 'Dinosaur' Glenn chasing skating gold in first Olympics
'Dinosaur' Glenn chasing skating gold in first Olympics
-
Gaza health officials say strikes kill 23 after Israel says shots wounded officer

-
 Italy foils Russian cyberattacks targeting Olympics
Italy foils Russian cyberattacks targeting Olympics
-
Stocks stabilise after Wall St AI-fuelled sell-off

-
 Figure skating favourite Malinin feeling 'the pressure' in Milan
Figure skating favourite Malinin feeling 'the pressure' in Milan
-
Netflix film probes conviction of UK baby killer nurse


Florence Connects 65,000+ Study Sites and 600+ Sponsors Worldwide - Unleashing the Next Era of Clinical Trial Intelligence
With the industry's largest network of sites, Florence sets a new benchmark for AI-enabled study startup, workflow automation, and operational risk management.
ATLANTA, GA / ACCESS Newswire / October 23, 2025 / Florence Healthcare reinforced its position as the global leader in clinical trial operations with a major milestone: Florence Trial Operations Platform now connects 65,000 study sites spanning more than 600 sponsors across 90+ countries, forming the industry's largest network. Recognized as the #1 clinical trial technology for six consecutive years, Florence continues to set the standard for startup speed, workflow automation, and operational risk management across sponsor study portfolios.
"Florence was built to bring sponsors and sites into a shared operational space," said Ryan Jones, CEO of Florence Healthcare. "First, we fully digitized startup to eliminate manual bottlenecks and paper workflows. Now, we're using AI to enhance speed and augment intelligence across 65,000 study sites globally - arming sponsors with portfolio-wide operational visibility so they can anticipate trial risk, act on insights, and drive execution on the ground."
Solving the $1 million per study problem
While digital-first sites set new standards for operational speed and efficiency, much of the industry still remains offline. Only 30% of global sites currently use an eISF, leaving roughly 200,000 sites dependent on inefficient paper processes for study startup, document collection and storage, regulatory process management, monitoring and closeout.
This lack of digitization costs sponsors an estimated $1 million per study in lost productivity, rework, quality and compliance risk.
Florence is closing this digital divide through the fastest growing clinical research network of 65,000 study sites and 600+ sponsors. By digitizing 100% of operational workflows, Florence sets the standard for startup speed, operational cost reduction, and risk control.
The results are compelling:
Up to 70% faster last mile study startup operations compared to the average site startup time for a Top 10 global pharmaceutical sponsor.
$141M annual operational cost takeout by automating site readiness workflows, based on active daily users and total hours saved per year across Florence's study site network.
51% increase in eTMF QA pass rates from 65% to 98.7%, due to built-in operational audits and automated compliance checks to control risk.
Shaping the Future of Study Operations
Florence is embedding AI across the study lifecycle to unlock operational data intelligence, recommend next-best actions, strengthen risk control, and improve operating efficiency.
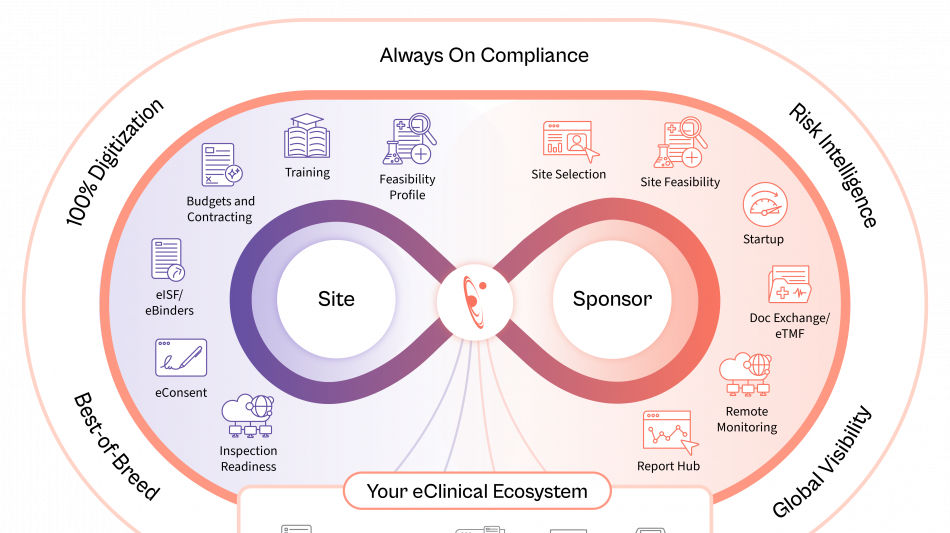
Site Identification & Feasibility
Drawing on the industry's largest network of site intelligence, Florence enables sponsors and CROs to identify the most qualified sites by therapeutic area, geography, and performance, while AI-assisted feasibility surveys ensure faster, more accurate completion.
Study Startup
AI-enabled contracting and document exchange between eTMF and eISF automates SSU document exchange, while generative AI redlining shortens contract review cycles, compares terms in seconds, and dramatically reduces time to activation.
Remote Monitoring
AI-powered reporting surfaces early risk signals from operational audit trails and site data, providing real-time visibility and enabling proactive intervention. These insights minimize the need for on-site visits and ensure trials stay on schedule.
Together, these advancements move sponsors and CROs from manual oversight to intelligent execution across global study portfolios. Through its open API network, Florence rapidly integrates and extends its trial operation capabilities to other eClinical partner systems.
Florence AI operates at the speed of trust, ensuring every study remains fully aligned with FDA, EMA, HIPAA, GDPR, EU Annex 11, ICH E6 (R3), and GCP standards.
All capabilities will be available in December 2025.
Join the Movement: Research Revolution 2025
Florence will showcase its newest capabilities at Research Revolution 2025, the company's annual global event (October 26-28, 2025) bringing together sponsors, CROs, and research sites. Be part of the global revolution. Watch it live at: https://researchrevolutionsummit.com/live/
About Florence Healthcare
Florence is a purpose-built platform that connects sponsors and sites to accelerate clinical trials, improve operational capacity, and reduce risk. Designed for scale, Florence streamlines workflows, enhances collaboration, and delivers real-time visibility across studies-empowering research teams to move faster, stay inspection-ready, and increase trial throughput with fewer resources.
Contact Information
Seema Sheth-Voss
[email protected]
(888) 829-0896
SOURCE: Florence Healthcare
View the original press release on ACCESS Newswire
J.Bergmann--BTB


