
-
 From 'flop' to Super Bowl favorite: Sam Darnold's second act
From 'flop' to Super Bowl favorite: Sam Darnold's second act
-
Man sentenced to life in prison for plotting to kill Trump in 2024

-
 Native Americans on high alert over Minneapolis crackdown
Native Americans on high alert over Minneapolis crackdown
-
Dallas deals Davis to Wizards in blockbuster NBA deal: report

-
 Russia 'no longer bound' by nuclear arms limits as treaty with US ends
Russia 'no longer bound' by nuclear arms limits as treaty with US ends
-
Panama hits back after China warns of 'heavy price' in ports row

-
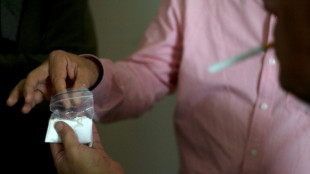 Strike kills guerrillas as US, Colombia agree to target narco bosses
Strike kills guerrillas as US, Colombia agree to target narco bosses
-
Wildfire smoke kills more than 24,000 Americans a year: study

-
 Telegram founder slams Spain PM over under-16s social media ban
Telegram founder slams Spain PM over under-16s social media ban
-
Curling kicks off sports programme at 2026 Winter Olympics

-
 Preventative cholera vaccination resumes as global supply swells: WHO
Preventative cholera vaccination resumes as global supply swells: WHO
-
Wales' Macleod ready for 'physical battle' against England in Six Nations

-
 Xi calls for 'mutual respect' with Trump, hails ties with Putin
Xi calls for 'mutual respect' with Trump, hails ties with Putin
-
'All-time great': Maye's ambitions go beyond record Super Bowl bid

-
 Shadow over Vonn as Shiffrin, Odermatt headline Olympic skiing
Shadow over Vonn as Shiffrin, Odermatt headline Olympic skiing
-
US seeks minerals trade zone in rare Trump move with allies

-
 Ukraine says Abu Dhabi talks with Russia 'substantive and productive'
Ukraine says Abu Dhabi talks with Russia 'substantive and productive'
-
Brazil mine disaster victims in London to 'demand what is owed'

-
 AI-fuelled tech stock selloff rolls on
AI-fuelled tech stock selloff rolls on
-
Russia vows to act 'responsibly' as nuclear pact ends with US

-
 White says time at Toulon has made him a better Scotland player
White says time at Toulon has made him a better Scotland player
-
Washington Post announces 'painful' job cuts

-
 All lights are go for Jalibert, says France's Dupont
All lights are go for Jalibert, says France's Dupont
-
Artist rubs out Meloni church fresco after controversy

-
 Palestinians in Egypt torn on return to a Gaza with 'no future'
Palestinians in Egypt torn on return to a Gaza with 'no future'
-
US removing 700 immigration officers from Minnesota

-
 Who is behind the killing of late ruler Gaddafi's son, and why now?
Who is behind the killing of late ruler Gaddafi's son, and why now?
-
Coach Thioune tasked with saving battling Bremen

-
 Russia vows to act 'responsibly' once nuclear pact with US ends
Russia vows to act 'responsibly' once nuclear pact with US ends
-
Son of Norway's crown princess admits excesses but denies rape

-
 US calls for minerals trade zone in rare move with allies
US calls for minerals trade zone in rare move with allies
-
Vowles dismisses Williams 2026 title hopes as 'not realistic'

-
 'Dinosaur' Glenn chasing skating gold in first Olympics
'Dinosaur' Glenn chasing skating gold in first Olympics
-
Gaza health officials say strikes kill 23 after Israel says shots wounded officer

-
 Italy foils Russian cyberattacks targeting Olympics
Italy foils Russian cyberattacks targeting Olympics
-
Stocks stabilise after Wall St AI-fuelled sell-off

-
 Figure skating favourite Malinin feeling 'the pressure' in Milan
Figure skating favourite Malinin feeling 'the pressure' in Milan
-
Netflix film probes conviction of UK baby killer nurse

-
 Timber hopes League Cup can be catalyst for Arsenal success
Timber hopes League Cup can be catalyst for Arsenal success
-
China calls EU 'discriminatory' over probe into energy giant Goldwind

-
 Sales warning slams Ozempic maker Novo Nordisk's stock
Sales warning slams Ozempic maker Novo Nordisk's stock
-
Can Vonn defy ACL rupture to win Olympic medal?

-
 Breakthrough or prelude to attack? What we know about Iran-US talks
Breakthrough or prelude to attack? What we know about Iran-US talks
-
German far-right MP detained over alleged Belarus sanctions breach

-
 MSF says its hospital in South Sudan hit by government air strike
MSF says its hospital in South Sudan hit by government air strike
-
Merz heads to Gulf as Germany looks to diversify trade ties

-
 Selection process for future Olympic hosts set for reform
Selection process for future Olympic hosts set for reform
-
Serbian minister on trial over Trump-linked hotel plan

-
 UK PM says Mandelson 'lied', regrets appointing him US envoy
UK PM says Mandelson 'lied', regrets appointing him US envoy
-
Cochran-Siegle tops first Olympic downhill training

| CMSD | -0.25% | 23.88 | $ | |
| SCS | 0.12% | 16.14 | $ | |
| CMSC | -0.51% | 23.54 | $ | |
| BCC | 5.97% | 90.32 | $ | |
| RIO | -0.05% | 96.32 | $ | |
| JRI | 0.04% | 13.125 | $ | |
| RBGPF | 0.12% | 82.5 | $ | |
| BCE | 1.08% | 26.385 | $ | |
| RYCEF | -2.41% | 16.6 | $ | |
| NGG | 2.36% | 88.31 | $ | |
| RELX | -1.8% | 29.97 | $ | |
| BTI | -0.11% | 61.805 | $ | |
| GSK | 7.09% | 57.41 | $ | |
| AZN | 1.92% | 187.92 | $ | |
| VOD | 2.96% | 15.715 | $ | |
| BP | 1.01% | 39.215 | $ |

Highly awaited Alzheimer's drug hit by delays
Eli Lilly's highly anticipated Alzheimer's drug has been held back for further review by regulators, the US pharmaceutical giant said Friday, in a blow for patients with the devastating brain disorder.
Donanemab has been found to slow cognitive decline in the early stages of the disease during a clinical trial -- but there was also a high rate of side effects, including deaths.
The Food and Drug Administration (FDA) "has informed Lilly it wants to further understand topics related to evaluating the safety and efficacy of donanemab," the company said in a statement Friday.
The regulator told the Indiana-based company it would convene a new meeting of experts, but hadn't provided a firm date. "As a result, the timing of expected FDA action on donanemab will be delayed beyond the first quarter of 2024."
"We are confident in donanemab's potential to offer very meaningful benefits to people with early symptomatic Alzheimer's disease," said Anne White, the company's executive vice president.
She added the FDA's decision to have a new meeting was "unexpected," but "We will work with the FDA and the stakeholders in the community to make that presentation and answer all questions."
Donanemab is an intravenously injected antibody that targets the build up beta-amyloid, a protein found in the brains of many patients with Alzheimer's.
Another anti-amyloid therapy called Leqembi, which was developed by Eisai of Japan and Biogen of Massachusetts, was granted full approval by the FDA last July and is now accessible through government-run health insurance for the elderly called Medicare.
- Slows decline, but risky -
In a paper published in the Journal of the American Medical Association last year, researchers found donanemab slowed cognitive and functional decline in patients who have early symptoms of the disease.
Forty-seven percent of those who received the drug showed no signs of cognitive decline after one year of treatment, compared to 29 percent who received a placebo.
Serious adverse events, including brain bleeds, occurred in 17.4 percent of those who received donanemab and 15.8 percent of those who received a placebo.
There were also four deaths: three in the donanemab group and one in the placebo group, but all the fatalities were considered a result of the treatment they received.
The trial recruited participants aged 60 to 85 with early symptomatic Alzheimer's, either mild cognitive impairment or Alzheimer's disease with mild dementia.
The news comes after the first Alzheimer's drug to be approved was pulled from the market in January.
The FDA awarded accelerated approval to Aduhelm in June 2021, a decision that was contentious at the time because the agency overruled its own independent advisors, who found there was insufficient evidence of benefit.
Biogen, which co-developed Aduhelm with Eisai, said it was discontinuing Aduhelm to focus its efforts of Leqembi.
Alzheimer's is the most common form of dementia. More than one in nine people over 65 develop the condition, which worsens over time, robbing them of their memories and independence, according to the US Alzheimer's Association.
K.Brown--BTB



