-
 Cuban tourism in crisis; visitors repelled by fuel, power shortages
Cuban tourism in crisis; visitors repelled by fuel, power shortages
-
Liverpool set for Jacquet deal, Palace sign Strand Larsen on deadline day

-
 FIFA president Infantino defends giving peace prize to Trump
FIFA president Infantino defends giving peace prize to Trump
-
Trump cuts India tariffs, says Modi will stop buying Russian oil

-
 Borthwick backs Itoje to get 'big roar' off the bench against Wales
Borthwick backs Itoje to get 'big roar' off the bench against Wales
-
Twenty-one friends from Belgian village win €123mn jackpot

-
 Mateta move to Milan scuppered by medical concerns: source
Mateta move to Milan scuppered by medical concerns: source
-
Late-January US snowstorm wasn't historically exceptional: NOAA

-
 Punctuality at Germany's crisis-hit railway slumps
Punctuality at Germany's crisis-hit railway slumps
-
Gazans begin crossing to Egypt for treatment after partial Rafah reopening

-
 Halt to MSF work will be 'catastrophic' for people of Gaza: MSF chief
Halt to MSF work will be 'catastrophic' for people of Gaza: MSF chief
-
Italian biathlete Passler suspended after pre-Olympics doping test

-
 Europe observatory hails plan to abandon light-polluting Chile project
Europe observatory hails plan to abandon light-polluting Chile project
-
Iran president orders talks with US as Trump hopeful of deal

-
 Uncertainty grows over when US budget showdown will end
Uncertainty grows over when US budget showdown will end
-
Oil slides, gold loses lustre as Iran threat recedes

-
 Russian captain found guilty in fatal North Sea crash
Russian captain found guilty in fatal North Sea crash
-
Disney earnings boosted by theme parks, as CEO handover nears

-
 Sri Lanka drop Test captain De Silva from T20 World Cup squad
Sri Lanka drop Test captain De Silva from T20 World Cup squad
-
France demands 1.7 bn euros in payroll taxes from Uber: media report

-
 EU will struggle to secure key raw materials supply, warns report
EU will struggle to secure key raw materials supply, warns report
-
France poised to adopt 2026 budget after months of tense talks

-
 Latest Epstein file dump rocks UK royals, politics
Latest Epstein file dump rocks UK royals, politics
-
Arteta seeks Arsenal reinforcement for injured Merino

-
 Russia uses sport to 'whitewash' its aggression, says Ukraine minister
Russia uses sport to 'whitewash' its aggression, says Ukraine minister
-
Chile officially backs Bachelet candidacy for UN top job

-
 European stocks rise as oil tumbles, while tech worries weigh on New York
European stocks rise as oil tumbles, while tech worries weigh on New York
-
England captain Itoje on bench for Six Nations opener against Wales

-
 Rahm says golfers should be 'free' to play where they want after LIV defections
Rahm says golfers should be 'free' to play where they want after LIV defections
-
More baby milk recalls in France after new toxin rules

-
 Rosenior will not rush Estevao return from Brazil
Rosenior will not rush Estevao return from Brazil
-
Mercedes ready to win F1 world title, says Russell

-
 Germany hit by nationwide public transport strike
Germany hit by nationwide public transport strike
-
Barca coach Flick 'not happy' with Raphinha thigh strain

-
 WHO chief says turmoil creates chance for reset
WHO chief says turmoil creates chance for reset
-
European stocks rise as gold, oil prices tumble

-
 Rink issues resolved, NHL stars chase Olympic gold at Milan
Rink issues resolved, NHL stars chase Olympic gold at Milan
-
S. Korea celebrates breakthrough K-pop Grammy win for 'Golden'

-
 Rodri rages that officials 'don't want' Man City to win
Rodri rages that officials 'don't want' Man City to win
-
Gaza's Rafah crossing makes limited reopening after two-year war

-
 African players in Europe: Ouattara dents Villa title hopes
African players in Europe: Ouattara dents Villa title hopes
-
Liverpool beat Chelsea to Rennes defender Jacquet - reports

-
 S. Korea celebrates breakthrough Grammy win for K-pop's 'Golden'
S. Korea celebrates breakthrough Grammy win for K-pop's 'Golden'
-
Trump says US talking deal with 'highest people' in Cuba

-
 Trump threatens legal action against Grammy host over Epstein comment
Trump threatens legal action against Grammy host over Epstein comment
-
Olympic Games in northern Italy have German twist

-
 Bad Bunny: the Puerto Rican phenom on top of the music world
Bad Bunny: the Puerto Rican phenom on top of the music world
-
Snapchat blocks 415,000 underage accounts in Australia

-
 At Grammys, 'ICE out' message loud and clear
At Grammys, 'ICE out' message loud and clear
-
Dalai Lama's 'gratitude' at first Grammy win

| SCS | 0.12% | 16.14 | $ | |
| CMSC | -0.04% | 23.75 | $ | |
| RIO | 1.4% | 92.325 | $ | |
| CMSD | 0.15% | 24.085 | $ | |
| AZN | -1.86% | 186.965 | $ | |
| RBGPF | 0.12% | 82.5 | $ | |
| BTI | 0.57% | 61.025 | $ | |
| NGG | -1.03% | 84.4 | $ | |
| GSK | 1.42% | 52.345 | $ | |
| BCC | 1.28% | 81.86 | $ | |
| RYCEF | 4.19% | 16.7 | $ | |
| BCE | -0.3% | 25.783 | $ | |
| JRI | 0.53% | 13.15 | $ | |
| RELX | -0.75% | 35.535 | $ | |
| BP | -0.37% | 37.74 | $ | |
| VOD | 1.88% | 14.93 | $ |

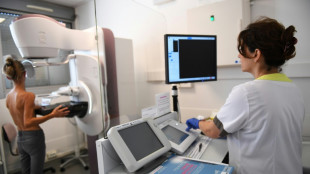
US health experts reassess hormone replacement therapy risks
US health authorities on Thursday began a reassessment of the risks surrounding Hormone Replacement Therapy (HRT), a treatment used by menopausal women around the world but long clouded by fear over its side effects.
HRT is taken to replace estrogen the body stops producing after menopause -- when periods end permanently -- and helps relieve symptoms such as hot flashes, vaginal discomfort, and pain during sex.
But its use has plummeted in recent years amid concerns including a potential link to invasive breast cancer.
Food and Drug Administration (FDA) chief Marty Makary, who convened Thursday's meeting of outside experts, has long advocated for HRT, saying its risks have been overstated.
"For decades, hormone replacement therapy for women -- that is estrogen or estrogen plus progesterone -- has helped women alleviate the symptoms of menopause, including hot flashes, dryness, mood swings, weight gain and poor sleep quality, to name a few," he said in a video ahead of the meeting.
He added that when initiated within a decade of the onset of the transitional period before menopause, HRT may even reduce cognitive decline, the risk of Alzheimer's, and prevent osteoporosis and cardiovascular disease.
Makary blamed the drop in HRT use on a landmark clinical trial, the Women's Health Initiative, which was halted in the early 2000s after it flagged increased risks of breast cancer and stroke. But he said subsequent studies had not replicated the findings on breast cancer.
"The many benefits of hormone therapy were ignored as it was seen as a carcinogen. Prescriptions for hormone replacement therapy plummeted in the United States, women flushed their pills down the toilet," he said Thursday.
"Fifty million plus women have not been offered the incredible potential health benefits of hormone replacement therapy because of medical dogma," he added, including his own mother, who suffered multiple bone fractures in her older life.
Critics of the trial argue it was flawed because the participants were too far from menopause, when risks are elevated and benefits limited, and that the formulations used are now outdated.
- Label changes -
Still, the issue remains divisive within the medical community.
The FDA's own warning label for HRT -- which can be administered through various means including orally, through skin patches, or vaginally -- cites risks including endometrial cancer, breast cancer, and life-threatening blood clots.
This week, the American Family Physician journal published an editorial that found limited benefits and significant harms associated with HRT.
"Menopause is a positive life experience for many women and should not be medicalized," the authors concluded.
The nature of the FDA expert meeting is also unusual. Unlike standard practice before the Trump administration, no agenda was publicly posted.
Several of the named panelists have ties to companies offering menopause treatments or who belong to the advocacy group "Let's Talk Menopause," which receives funding from pharmaceutical companies and campaigns to revise the FDA warning label.
J.Horn--BTB