
-
 Trump signs order reclassifying marijuana as less dangerous
Trump signs order reclassifying marijuana as less dangerous
-
Famed Kennedy arts center to be renamed 'Trump-Kennedy Center'

-
 US accuses S.Africa of harassing US officials working with Afrikaners
US accuses S.Africa of harassing US officials working with Afrikaners
-
Brazil open to EU-Mercosur deal delay as farmers protest in Brussels

-
 Wounded Bangladesh youth leader dies in Singapore hospital
Wounded Bangladesh youth leader dies in Singapore hospital
-
New photo dump fuels Capitol Hill push on Epstein files release

-
 Brazil, Mexico seek to defuse US-Venezuela crisis
Brazil, Mexico seek to defuse US-Venezuela crisis
-
Assange files complaint against Nobel Foundation over Machado win

-
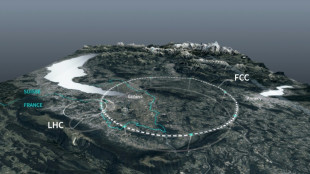 Private donors pledge $1 bn for CERN particle accelerator
Private donors pledge $1 bn for CERN particle accelerator
-
Russian court orders Austrian bank Raiffeisen to pay compensation

-
 US, Qatar, Turkey, Egypt to hold Gaza talks in Miami
US, Qatar, Turkey, Egypt to hold Gaza talks in Miami
-
Lula open to mediate between US, Venezuela to 'avoid armed conflict'

-
 Brussels farmer protest turns ugly as EU-Mercosur deal teeters
Brussels farmer protest turns ugly as EU-Mercosur deal teeters
-
US imposes sanctions on two more ICC judges for Israel probe

-
 US accuses S. Africa of harassing US officials working with Afrikaners
US accuses S. Africa of harassing US officials working with Afrikaners
-
ECB holds rates as Lagarde stresses heightened uncertainty

-
 Trump Media announces merger with fusion power company
Trump Media announces merger with fusion power company
-
Stocks rise as US inflation cools, tech stocks bounce

-
 Zelensky presses EU to tap Russian assets at crunch summit
Zelensky presses EU to tap Russian assets at crunch summit
-
Pope replaces New York's Cardinal Dolan with pro-migrant bishop

-
 Odermatt takes foggy downhill for 50th World Cup win
Odermatt takes foggy downhill for 50th World Cup win
-
France exonerates women convicted over abortions before legalisation

-
 UK teachers to tackle misogyny in classroom
UK teachers to tackle misogyny in classroom
-
Historic Afghan cinema torn down for a mall

-
 US consumer inflation cools unexpectedly in November
US consumer inflation cools unexpectedly in November
-
Danish 'ghetto' residents upbeat after EU court ruling

-
 ECB holds rates but debate swirls over future
ECB holds rates but debate swirls over future
-
Pope replaces New York's Cardinal Timothy Dolan with little-known bishop

-
 Bank of England cuts interest rate after UK inflation slides
Bank of England cuts interest rate after UK inflation slides
-
Have Iran's authorities given up on the mandatory hijab?

-
 Spain to buy 100 military helicopters from Airbus
Spain to buy 100 military helicopters from Airbus
-
US strike on alleged drug boat in Pacific kills four

-
 Thailand strikes building in Cambodia's border casino hub
Thailand strikes building in Cambodia's border casino hub
-
Protests in Bangladesh as India cites security concerns

-
 European stocks rise before central bank decisions on rates
European stocks rise before central bank decisions on rates
-
Tractors clog Brussels in anger at EU-Mercosur trade deal

-
 Not enough evidence against Swedish PM murder suspect: prosecutor
Not enough evidence against Swedish PM murder suspect: prosecutor
-
Nepal's ousted PM Oli re-elected as party leader

-
 British energy giant BP extends shakeup with new CEO pick
British energy giant BP extends shakeup with new CEO pick
-
Pulitzer-winning combat reporter Peter Arnett dies at 91

-
 EU kicks off crunch summit on Russian asset plan for Ukraine
EU kicks off crunch summit on Russian asset plan for Ukraine
-
Lyon humbled to surpass childhood hero McGrath's wicket tally

-
 Sri Lanka plans $1.6 bn in cyclone recovery spending in 2026
Sri Lanka plans $1.6 bn in cyclone recovery spending in 2026
-
England vow to keep 'fighting and scrapping' as Ashes slip away

-
 'Never enough': Conway leans on McKenzie wisdom in epic 300 stand
'Never enough': Conway leans on McKenzie wisdom in epic 300 stand
-
Most Asian markets track Wall St lower as AI fears mount

-
 Cambodia says Thailand bombs casino hub on border
Cambodia says Thailand bombs casino hub on border
-
Thai queen wins SEA Games gold in sailing

-
 England Ashes dreams on life-support as Australia rip through batting
England Ashes dreams on life-support as Australia rip through batting
-
Masterful Conway, Latham in 323 opening stand as West Indies wilt

| RYCEF | 3.97% | 15.38 | $ | |
| CMSC | 0.09% | 23.28 | $ | |
| RBGPF | -2.23% | 80.22 | $ | |
| NGG | -0.74% | 76.592 | $ | |
| BP | -3.12% | 33.428 | $ | |
| AZN | 1.23% | 90.975 | $ | |
| RELX | 0.42% | 40.73 | $ | |
| RIO | 0.68% | 77.72 | $ | |
| BTI | 0.08% | 57.215 | $ | |
| GSK | -0.54% | 48.45 | $ | |
| SCS | 0.12% | 16.14 | $ | |
| VOD | 0.16% | 12.83 | $ | |
| CMSD | 0.09% | 23.3 | $ | |
| JRI | -0.09% | 13.418 | $ | |
| BCC | 1.62% | 77.55 | $ | |
| BCE | -1.11% | 22.895 | $ |

BioNxt Launches Feasibility Study for Semaglutide Oral Thin Film as Alternative to Injection/Tablets
VANCOUVER, BC / ACCESS Newswire / June 23, 2025 / BioNxt Solutions Inc. ("BioNxt" or the "Company") (CSE:BNXT)(OTC:BNXTF)(FSE:BXT), is pleased to announce the launch of a feasibility study for the development of an oral dissolvable film (ODF) formulation of Semaglutide, a GLP-1 receptor agonist currently marketed globally under the brand names Ozempic®, Rybelsus®, and Wegovy®.
This initiative marks a significant step in evaluating BioNxt's proprietary thin film drug delivery platform as a non-invasive, user-friendly alternative to injectable and tablet-based GLP-1 therapies. The study is designed to assess the compatibility of Semaglutide with BioNxt's ODF technology and generate early data to inform formulation optimization and intellectual property strategy.
"We believe there is a compelling opportunity to rethink how complex molecules like Semaglutide are delivered," said Hugh Rogers, CEO at BioNxt. "Our oral thin film platform is engineered to improve patient adherence and comfort, and this feasibility study is an important first step in bringing a new delivery option to market."
Semaglutide: A High-Growth Market Segment
Semaglutide is a GLP-1 analog that has rapidly become a blockbuster drug for type 2 diabetes and obesity, with growing use in cardiovascular risk reduction. According to a 2024 report by Data Bridge Market Research, the global GLP‑1 receptor agonist market was valued at USD 24.4 billion in 2023 and is projected to reach approximately USD 156.7 billion by 2030, driven by increasing obesity rates and the rising demand for non‑insulin therapies.
Despite its success, most current Semaglutide formulations still require weekly injections or large daily oral tablets, which can affect patient adherence. A rapidly dissolving oral thin film promises a significant boost in accessibility, convenience, and overall patient experience.
Study Objectives
The feasibility program includes:
Pre-formulation screening and platform compatibility assessment
Drug loading and film integrity evaluation for peptide stability
Generation of early-stage technical data to support patent filings
The feasibility study is currently in the planning phase, with receipt of the Semaglutide active pharmaceutical ingredient (API) anticipated in July. Upon delivery, pre-formulation activities and laboratory-scale formulation trials will be initiated. Subsequent prototype development and characterization are expected to support a provisional patent filing in the third quarter of 2025.
This project represents a strategic expansion of BioNxt's oral thin film (ODF) drug delivery platform, which is designed to enable the non-invasive administration of high-value therapeutics. Semaglutide is the first in a planned series of GLP-1 receptor agonists being developed using this technology. The platform is particularly well-suited for peptide-based compounds and holds significant potential to support additional thin film solutions across the rapidly growing class of GLP-1 therapies targeting diabetes, obesity, and related metabolic disorders.
About BioNxt Solutions Inc.
BioNxt Solutions Inc. is a bioscience innovator focused on next-generation drug delivery technologies, diagnostic screening systems, and active pharmaceutical ingredient development. The Company's proprietary platforms - Sublingual (Thin-Film), Transdermal (Skin Patch), and Oral (Enteric-Coated Tablets) - target key therapeutic areas, including autoimmune diseases, neurological disorders, and longevity.
With research and development operations in North America and Europe, BioNxt is advancing regulatory approvals and commercialization efforts, primarily focused on European markets. BioNxt is committed to improving healthcare by delivering precise, patient-centric solutions that enhance treatment outcomes worldwide.
BioNxt is listed on the Canadian Securities Exchange: BNXT, OTC Markets: BNXTF and trades in Germany under WKN: A3D1K3. To learn more about BioNxt, please visit www.bionxt.com.
Investor Relations & Media Contact
Hugh Rogers, Co-Founder, CEO and Director
Email: [email protected]
Phone: +1 778.598.2698
Web: www.bionxt.com
LinkedIn: https://www.linkedin.com/company/bionxt-solutions
Instagram: https://www.instagram.com/bionxt
Cautionary Statement Regarding "Forward-Looking" Information
This press release contains "forward-looking information" and "forward-looking statements" within the meaning of applicable Canadian securities laws (collectively, "forward-looking information"). Forward-looking information includes, but is not limited to, statements related to the Company's oral thin film (ODF) development program for Semaglutide; the anticipated timing and outcomes of formulation studies; the potential for future patent filings; market growth projections; and the broader applicability of the Company's drug delivery technologies.
Forward-looking information is based on management's reasonable assumptions, expectations, estimates, and projections as of the date of this press release. Such statements are subject to various known and unknown risks, uncertainties, and other factors - many of which are beyond the Company's control - that may cause actual results, performance, or achievements to differ materially from those expressed or implied by such forward-looking information. These risks and uncertainties include, but are not limited to: scientific and technical development risks; manufacturing and scalability risks; intellectual property protection; regulatory approval processes; competition in the GLP-1 drug market; and general economic, financial, and market conditions.
Readers are cautioned not to place undue reliance on forward-looking information. Although the Company believes the expectations and assumptions reflected in such statements are reasonable, there can be no assurance that they will prove to be correct. Except as required by applicable securities laws, the Company undertakes no obligation to update or revise any forward-looking information, whether as a result of new information, future events, or otherwise.
Ozempic®, Rybelsus®, and Wegovy® are registered trademarks of Novo Nordisk A/S and are not affiliated with or developed by BioNxt Solutions Inc.
SOURCE: BioNxt Solutions Inc.
View the original press release on ACCESS Newswire
A.Gasser--BTB
