-
 All lights are go for Jalibert, says France's Dupont
All lights are go for Jalibert, says France's Dupont
-
Artist rubs out Meloni church fresco after controversy

-
 Palestinians in Egypt torn on return to a Gaza with 'no future'
Palestinians in Egypt torn on return to a Gaza with 'no future'
-
US removing 700 immigration officers from Minnesota

-
 Who is behind the killing of late ruler Gaddafi's son, and why now?
Who is behind the killing of late ruler Gaddafi's son, and why now?
-
Coach Thioune tasked with saving battling Bremen

-
 Russia vows to act 'responsibly' once nuclear pact with US ends
Russia vows to act 'responsibly' once nuclear pact with US ends
-
Son of Norway's crown princess admits excesses but denies rape

-
 US calls for minerals trade zone in rare move with allies
US calls for minerals trade zone in rare move with allies
-
Vowles dismisses Williams 2026 title hopes as 'not realistic'

-
 'Dinosaur' Glenn chasing skating gold in first Olympics
'Dinosaur' Glenn chasing skating gold in first Olympics
-
Gaza health officials say strikes kill 23 after Israel says shots wounded officer

-
 Italy foils Russian cyberattacks targeting Olympics
Italy foils Russian cyberattacks targeting Olympics
-
Stocks stabilise after Wall St AI-fuelled sell-off

-
 Figure skating favourite Malinin feeling 'the pressure' in Milan
Figure skating favourite Malinin feeling 'the pressure' in Milan
-
Netflix film probes conviction of UK baby killer nurse

-
 Timber hopes League Cup can be catalyst for Arsenal success
Timber hopes League Cup can be catalyst for Arsenal success
-
China calls EU 'discriminatory' over probe into energy giant Goldwind

-
 Sales warning slams Ozempic maker Novo Nordisk's stock
Sales warning slams Ozempic maker Novo Nordisk's stock
-
Can Vonn defy ACL rupture to win Olympic medal?

-
 Breakthrough or prelude to attack? What we know about Iran-US talks
Breakthrough or prelude to attack? What we know about Iran-US talks
-
German far-right MP detained over alleged Belarus sanctions breach

-
 MSF says its hospital in South Sudan hit by government air strike
MSF says its hospital in South Sudan hit by government air strike
-
Merz heads to Gulf as Germany looks to diversify trade ties

-
 Selection process for future Olympic hosts set for reform
Selection process for future Olympic hosts set for reform
-
Serbian minister on trial over Trump-linked hotel plan

-
 UK PM says Mandelson 'lied', regrets appointing him US envoy
UK PM says Mandelson 'lied', regrets appointing him US envoy
-
Cochran-Siegle tops first Olympic downhill training

-
 Gaza health officials say strikes kill 21 after Israel says shots wounded officer
Gaza health officials say strikes kill 21 after Israel says shots wounded officer
-
Injured Vonn's Olympic bid is 'inspirational', ski stars say

-
 Albania arrests 20 for toxic waste trafficking
Albania arrests 20 for toxic waste trafficking
-
US-Africa trade deal renewal only 'temporary breather'

-
 Mir sets pace on Sepang day two, Yamaha absent
Mir sets pace on Sepang day two, Yamaha absent
-
Xi, Putin hail 'stabilising' China-Russia alliance

-
 GSK boosted by specialty drugs, end to Zantac fallout
GSK boosted by specialty drugs, end to Zantac fallout
-
UK's ex-prince leaves Windsor home amid Epstein storm: reports

-
 Sky is the limit for Ireland fly-half Prendergast, says captain Doris
Sky is the limit for Ireland fly-half Prendergast, says captain Doris
-
Stocks fluctuate after Wall St AI-fuelled sell-off

-
 Feyi-Waboso reminds England great Robinson of himself
Feyi-Waboso reminds England great Robinson of himself
-
Starmer faces MPs as pressure grows over Mandelson scandal

-
 HRW urges pushback against 'aggressive superpowers'
HRW urges pushback against 'aggressive superpowers'
-
Russia demands Ukraine give in as UAE talks open

-
 Gaza civil defence says 17 killed in strikes after Israel says shots wounded officer
Gaza civil defence says 17 killed in strikes after Israel says shots wounded officer
-
France's Kante joins Fenerbahce after Erdogan 'support'

-
 CK Hutchison launches arbitration over Panama Canal port ruling
CK Hutchison launches arbitration over Panama Canal port ruling
-
Stocks mostly rise as traders ignore AI-fuelled sell-off on Wall St

-
 Acclaimed Iraqi film explores Saddam Hussein's absurd birthday rituals
Acclaimed Iraqi film explores Saddam Hussein's absurd birthday rituals
-
On rare earth supply, Trump for once seeks allies

-
 Ukrainian chasing sumo greatness after meteoric rise
Ukrainian chasing sumo greatness after meteoric rise
-
Draper to make long-awaited return in Davis Cup qualifier


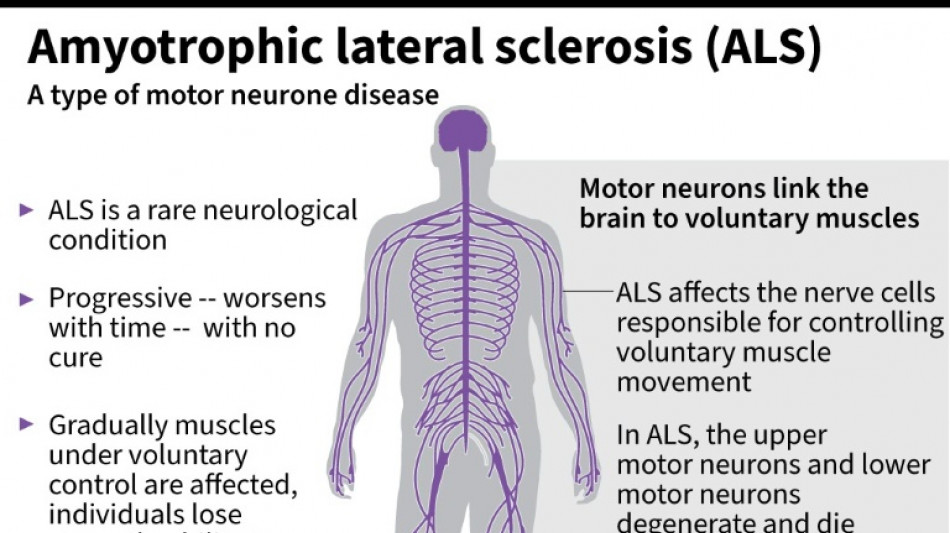
US company withdraws ALS drug after it fails in trial
Amylyx Pharmaceuticals announced Thursday it was withdrawing its approved treatment against the deadly neurodegenerative disease ALS after clinical data found no evidence the drug worked.
In a statement, the US company said it would discontinue its market authorizations for Relyvrio/Albrioza, using the brand names of the medicine in the US and Canadian markets.
"While this is a difficult moment for the ALS community, we reached this path forward in partnership with the stakeholders who will be impacted and in line with our steadfast commitment to people living with ALS and other neurodegenerative diseases," said the company's co-CEOs Joshua Cohen and Justin Klee in a statement.
The company also said it was reducing its workforce "by approximately 70 percent" as it focused on another experimental drug for use against ALS, and on repurposing Relyvrio for other conditions. It added it would continue to make Relyvrio available for patients who wish to keep using the treatment, through a "free drug program."
The news follows data from a clinical trial of 664 ALS patients announced in March, which found no significant differences in outcomes between those on the treatment group and those who received a placebo.
It was a big blow for patients with amyotrophic lateral sclerosis, sometimes called Lou Gehrig's disease after the famous baseball player, which devastates nerve cells in the brain and spinal cord.
ALS affects about two people per 100,000 every year, causing progressive loss of motor and cognitive function. Most patients die within five years of their diagnosis.
Relyvrio's approval by the US Food and Drug Administration in 2022 was controversial and based on the results of a single trial that involved just 137 participants.
The FDA itself noted there was "residual uncertainty about the evidence of effectiveness" -- but "given the serious and life-threatening nature of ALS and the substantial unmet need, this level of uncertainty is acceptable in this instance and consideration of these results in the context of regulatory flexibility is appropriate."
- Patient groups backed approval -
Advocacy groups also mounted a major campaign sending a petition to the FDA with tens of thousands of signatures urging approval. Once it became available, Amylyx reportedly announced an eye-watering list price of $158,000 per year in the US, drawing criticism.
Patient groups in Europe watched with desperation at the bureaucratic delays.
When the European Union drug watchdog later announced it was rejecting Relyvrio, the decision was slammed as "an affront" by angry French patients, who say they "don't have time to wait." France later relented, offering conditional approval in November.
"We commend Amylyx for pulling Relyvrio off the market, while still ensuring that people living with ALS can access the drug if they believe it is helping them," said the US-based ALS association, which had lobbied for the drug's approval and funded its research.
"Safe and potentially effective treatments can be made accessible rapidly until further research can confirm their efficacy," it added.
For now, there remain only a handful of treatments available.
Riluzole, FDA approved in 1995, prolongs life approximately three months. Edaravone, FDA approved in 2017, has been found to slow disease progression and improve survival.
And in 2023, the regulatory body approved tofersen, a gene therapy treatment that targets those ALS cases that are caused by mutations in the SOD1 gene.
K.Thomson--BTB




